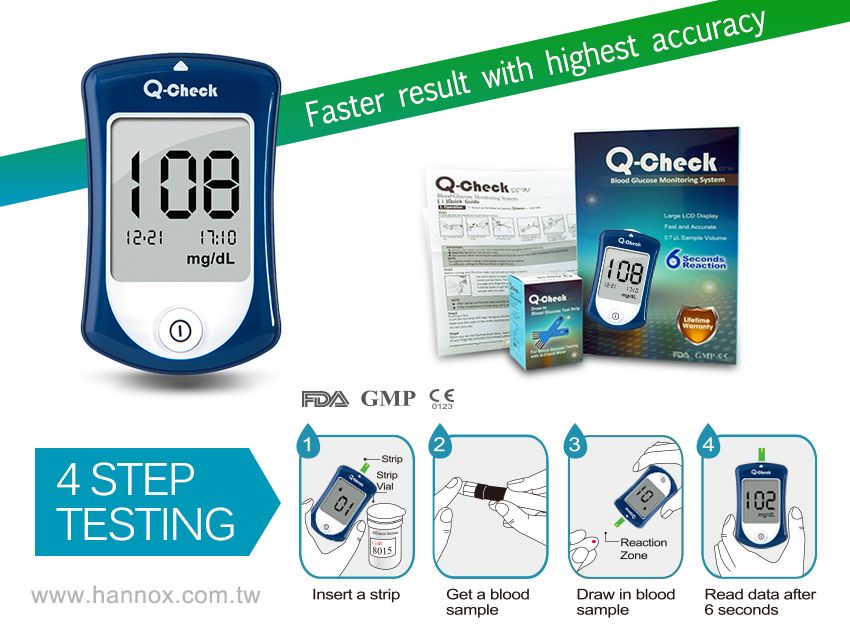
Q-check Blood Glucose Monitoring System is FDA cleared, and meets GMP, ISO 13485 and CE mark standard.
Performing a glucose test with Q-check, you have different options of testing parts on your body besides the fingertip like palms or forearms as an alternative.
* Texas instruments chip inside
* Coding-Free
* Anti-Bacteria surface

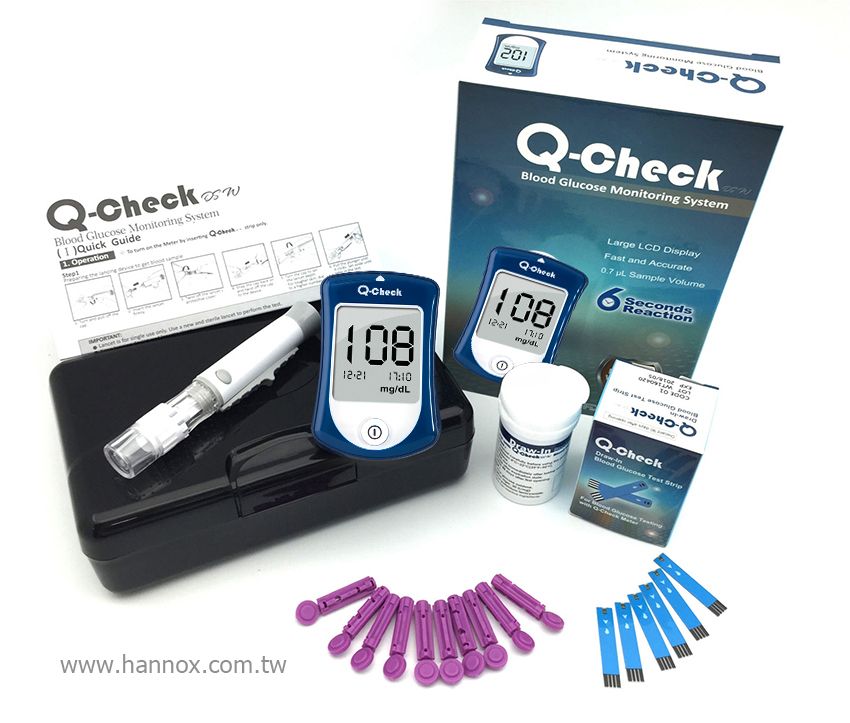
OEM/ODM Cooperation is Welcomed
Tell us your demand, let's work together to make your product come ture and hit the market successfully.
| Assay Range | 20 to 600 mg / dl (11.1 to 33.3 mmol/L) |
|---|---|
| Accuracy | 90% in zone A of EGA |
| Sample Volume | 0.7µL |
| Reaction Time | 6 seconds |
| Operational Temperature | 10 to 40°C ( 50 to 104°F) |
| Relative Humidity | 10 to 90% |
| Hematocrit | 20 to 60% |
| Battery Type | CR2032 3V lithium coin battery |
| Dimension | 84 x 55 x 13 mm |
| Weight | 42.8g without battery |
| Certificate | FDA, GMP, CE0123 |
Taiwantrade.com and iDealEZ.com uses analytical cookies and other tracking technologies to offer you the best possible user experience. By using our website, you acknowledge and agree to our cookie policy.
For more information on cookies or changing your cookies settings, read Taiwantrade & iDealEZ’s Privacy Policy.